Your starting cost of paper for PROs is estimated to be:
$…
You submitted the following information:
… WEEK(S)
Study duration
…
Number of study participants
…
Frequency of data collection
…
Number of pages per assessment
1 assessment is equal to 2 pages
Interested to know how this compares to eCOA?
Click the button below and our team will promptly review this information to initiate a personalized review with one of our scientists aimed at enhancing your trial outcomes and reducing that cost.
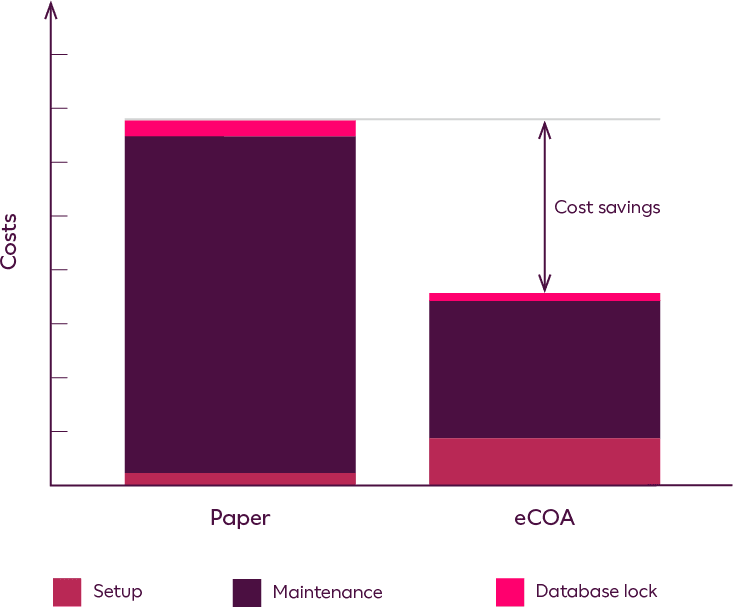
It’s not just about direct costs.
While traditional paper-based methods may seem more affordable at first glance, the costs associated with paper-based COA solutions often accumulate over time, equaling or even exceeding the overall expenses of electronic COA (eCOA) solutions. Those costs include:
Non-compliance
eCOA non-compliance is often called “parking lot syndrome”, where patients rush to fill out assessments just before visits. This undermines the data’s reliability and compromises the integrity of clinical trial results.
Paper Diary Design
Paper diary design is flawed as it can lead to inaccurate data due to patient recall bias, inconsistent entry times, and the potential for lost or incomplete records.
Data Transcription
Increased potential for errors during manual entry, the additional time required for data verification, and the subsequent delays in study timelines and decision-making.
Paper Diary Review by Study Coordinator
Increases the burden on staff, while potentially compromising PHI and data integrity.
Source Data Verification by CRA
Resource-intensive and can introduce delays and additional costs to clinical trials due to the extensive manual cross-checking required.
Data Management
Not only the direct expenses of software and staffing but also the indirect costs associated with data integration, long-term data storage, ensuring regulatory compliance, and the potential need for specialized training for personnel.
Query Generation
Having to run query generation indicates potential data inconsistencies or errors, necessitating additional review and clarification, which can lead to increased administrative burden, delays in study timelines, and additional costs.
Query Resolution
Demands valuable time and resources for data clarification, potential delays in the study timeline, and increased workload for clinical staff.
Shipment of Paper COAs to/from the Site
More than just direct mailing expenses, but also potential delays in data availability and analysis and the risk of loss or damage to data.
Archiving of Paper Assessments
Makes the assessments vulnerable to physical damage, loss, unauthorized access, and the challenges of long-term storage and retrieval, which can jeopardize the integrity and availability of crucial trial data.
Translations and Licensing
Substantial fees for professional translation services and ongoing licensing fees for paper COA systems.
Environmental Impact
Considering that an average tree yields 16.67 reams of paper, this also contributes to deforestation and increases to carbon footprint from paper production and transportation.
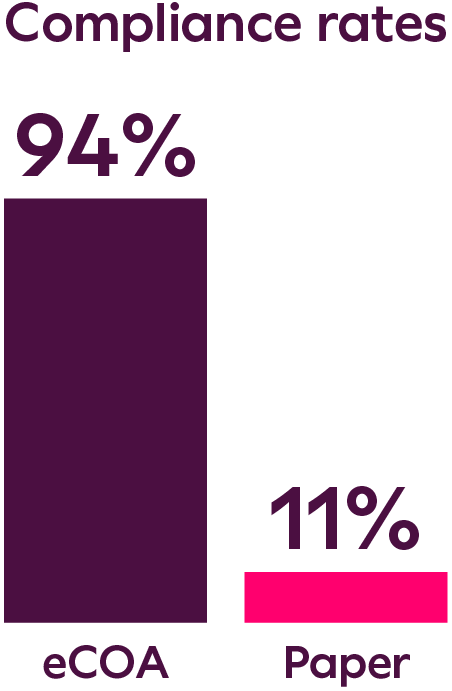
Compliance rates fall with paper*
While patients completed paper diaries in over 90% of instances, only 11% of those completions occurred at the intended time, indicating a significant gap in actual compliance.
Data integrity is often very costly
Paper isn’t time stamped – are the patients doing the questionnaires when required for the study or are they doing it in the car before they enter the facility?
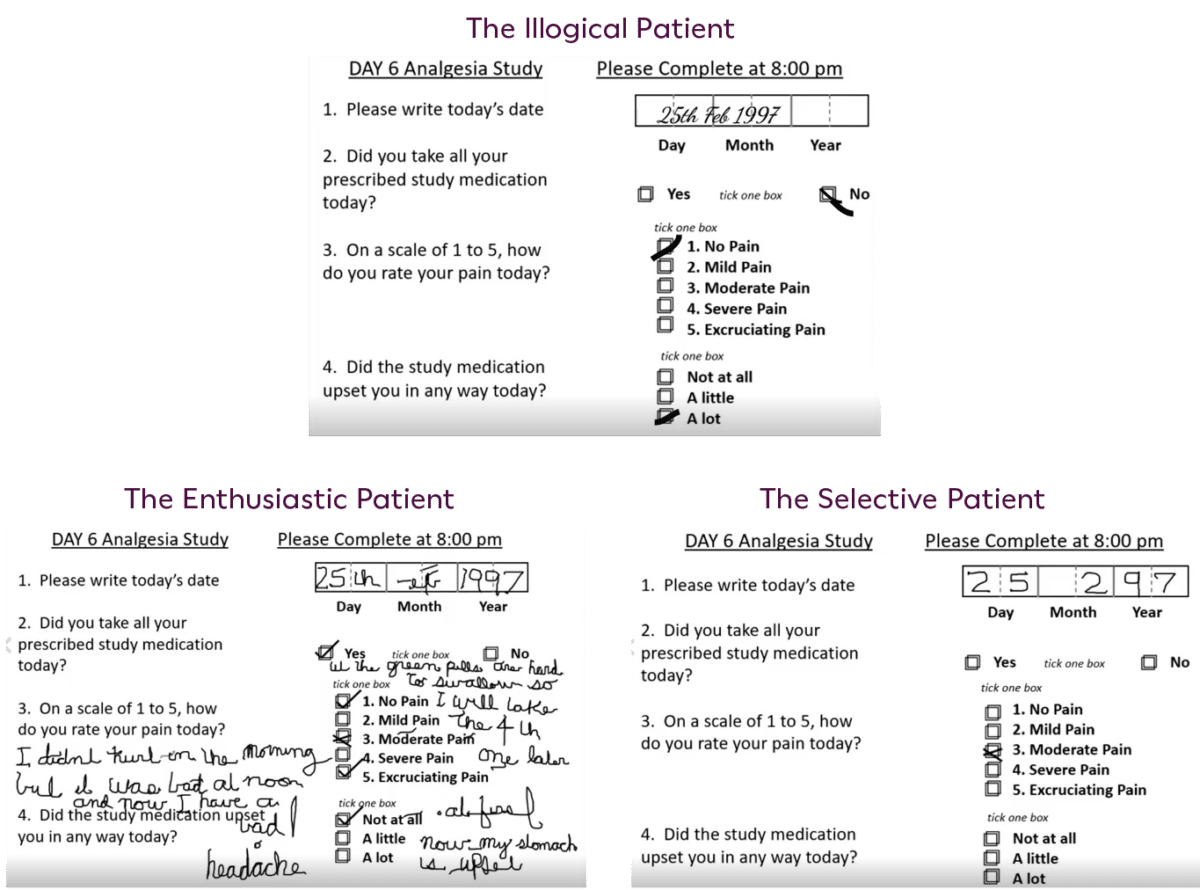
Paper
vs
Electronic
Unlock hidden savings with eCOA
Traditional paper assessments can be a silent drain on your resources after all of the unseen costs. With eCOA, you gain more than just a streamlined data collection process; you invest in a comprehensive solution that eliminates unseen costs and optimizes your resources.
*Stone et al,. Patient non-compliance with paper diaries, BMJ, 2002, Vol 324